Fluid Thoughts
- Meissner/FujiFilm’s ATF video presentation at INTERPHEX NYMeissner’s Unique Approach to Characterizing Its Ultrafiltration Membranes Results in Lot-to-Lot Performance Consistency
- Better Characterized Ultrafiltration Membranes to Improve BiomanufacturingMeissner’s Unique Approach to Characterizing Its Ultrafiltration Membranes Results in Lot-to-Lot Performance Consistency
- Transforming Biocontainer Mixing: Meissner’s Collaborative RMS ApproachUpgrade your biomanufacturing operation with confidence: Meissner’s retrofit RMS mixing solutions empower Mobius Mix users to secure reliable, high-performance mixing—backed by Meissner’s trusted supply resilience and applications engineering. Discover how our innovative approach transforms risk into opportunity for biomanufacturers who demand more than just mixing.
- Meissner Presents New Way to Characterize Ultrafiltration MembranesWatch our presentation at the Biotech Week Boston 2023 conference on “An Improved Method of Characterization of Ultrafiltration (UF) Membranes for Bioprocessing”, given by Thomas D. Lazzara, Ph.D., Director of Research and Development. The characterization of ultrafiltration membranes with molecular weight (Mw) markers is a standard method to determine the retention rating of membranes in
- Technical Theater Presentations at INTERPHEX New York 2023Meissner delivered two product portfolio updates during a Technical Theater presentation at the INTERPHEX New York 2023 event. The presentations included a bench top freezer addition to the CryoVault® freeze & thaw platform for critical biopharmaceuticals and the expansion of the SepraPor® hollow fiber filter portfolio to include ultrafiltration applications.
- Meissner Filtration Products Has Shortened Its Name to MeissnerFor the last 40 years, Meissner has been expanding its expertise in the pharma and biotech industries beyond filtration into single-use systems, aseptic filling systems, freeze & thaw platforms for critical biopharmaceuticals, and laboratory and technical services. To more closely align with the way our clients refer to us, and all of the critical manufacturing solutions we offer, Meissner has officially shortened its name from “Meissner Filtration Products” to “Meissner.”
- Meissner Announces Location of Future Manufacturing Site in Athens, Georgia!Meissner Corporation, a leading manufacturer of advanced microfiltration and single-use systems for production of pharmaceutical drugs, biologics, and cell and gene therapies, will invest nearly $250 million in a new manufacturing facility in Athens, Georgia.
- Biotech Week Boston/BPI Virtual Event 2020 Interview – “What’s New at Meissner?”The BPI US virtual event will run September 21-24, 2020. The presentation can be viewed On-Demand through the Live Labs section on the virtual portal. A free Exhibit Pass to the event can be requested by visiting https://bit.ly/2FIrLvN. Meissner will be hosting a Solutions Provider booth where visitors can video chat with our team and download product information.
- Single-Use Roundtable Discussion is Featured in American Pharmaceutical Review MagazineThe magazine explores the opinions of 14 prominent industry figures centering around Single-Use adoption, advantages/disadvantages, and the future outlook.
- Meissner’s CryoVault® Freeze & Thaw SolutionA Closer Look at the CryoVault® Platform for Freeze and Thaw of Bulk Drug Substance (BDS) and Critical Biopharmaceuticals.
- Meissner Announces Its New European Manufacturing Facility in Castlebar, IrelandAt a press conference on Friday, 01FEB2019, Meissner announced that it will expand manufacturing operations by establishing a 100,000 sq.ft. facility in County Mayo, Ireland, creating upwards of 150 jobs over the next 5 years.
- Catch Jeff Johnson, of Merck & Co., Inc., as he delivers a video presentation on a Large Scale BDS Freeze & Thaw PlatformJeff Johnson, Director and New Technology Lead, Global Engineering Solutions, Merck & Co., Inc., delivers a live presentation at INTERPHEX New York on a “Large Scale Single-Use Bulk Drug Substance Freeze and Thaw Platform.” This presentation is an overview of a novel single-use end-to-end solution for freeze, thaw, handling and transport of Bulk Drug Substance. Jeff’s talk touches on design consideration, scalability, and stability.
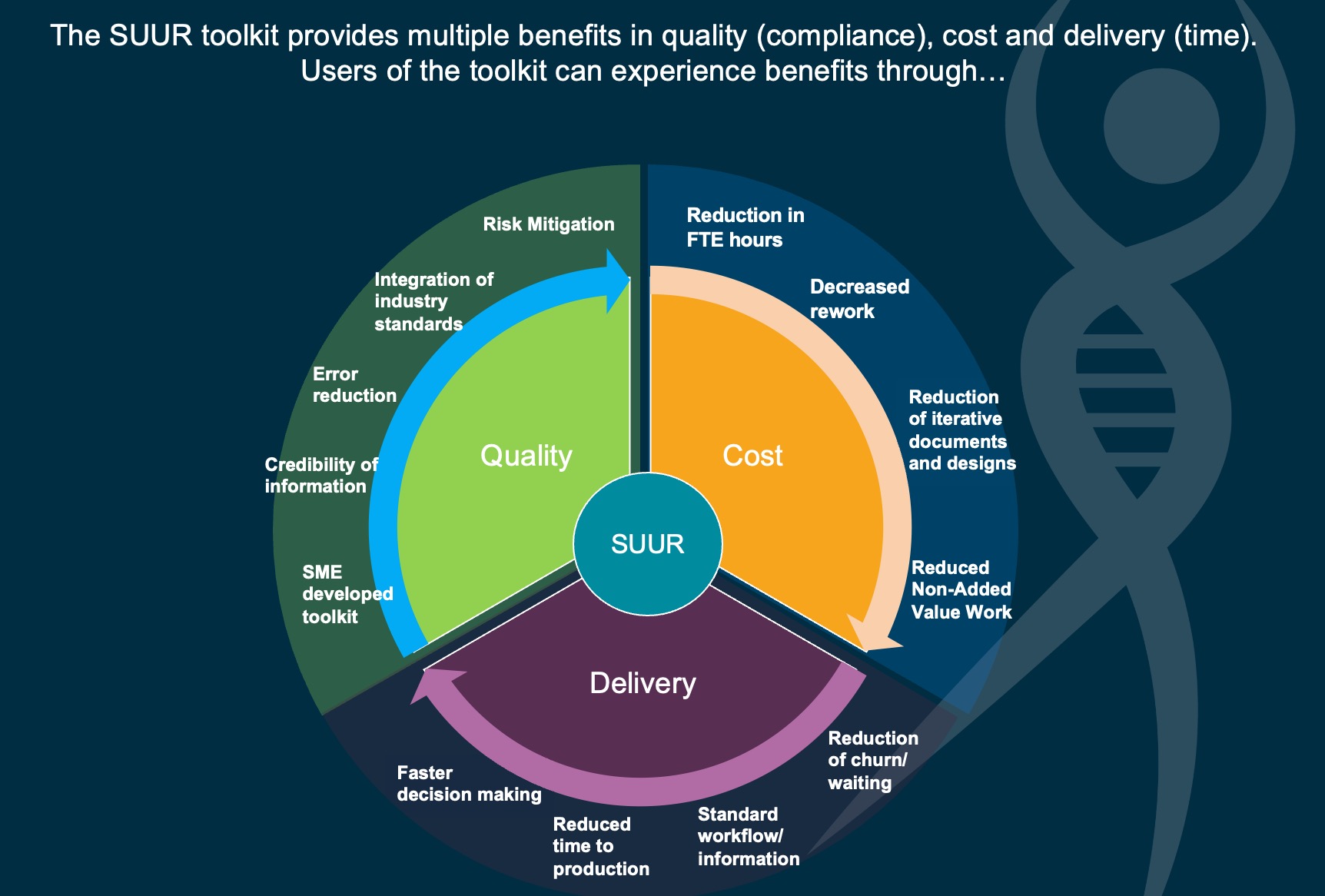
- The Benefits of using the BioPhorum and BPSA’s co-developed SUUR (Single-Use User Requirements) ToolkitJoin Christian Julien, Meissner Filtration Products and Danielle Werner, Merck, as they discuss the benefits of using the BioPhorum and BPSA’s co-developed SUUR Toolkit in this short video, led by Roberta Toporovski, Bristol-Meyers Squibb.
- Single-Use Filling Assemblies Interphex VideoMax Blomberg, Director of Operations, addresses some of the unique challenges associated with fill and finish operations and how Meissner has worked to mitigate them.
- The CryoVault® Freeze and Thaw Platform for BiopharmaceuticalsAndrew Govea, Product Engineer, introduces us to this new technology platform and explains the four modular components live at INTERPHEX New York.
- Meissner Acquires an Aseptic Filling CompanyCamarillo, CA, January 4th, 2018 – Meissner Filtration products announced that it has acquired PDC Aseptic Filling Systems, a supplier of advanced aseptic filling systems and sealers to the pharmaceutical industry.
- Conductivity Testing of QuaDrum® Mixing SystemsThis technical bulletin highlights the results of conductivity testing to characterize recirculation mixing performance of single-use systems deployed in Meissner’s QuaDrum® rigid outer containers (ROCs).
- Recirculation Flow Testing a FlexGro® BiocontainerRocker bioreactors, also commonly referred to as wave bioreactors, have been a popular choice for seed train applications and small scale protein production for required working volumes below the 100 L range.
- Scale-up by Scaling OutThere is a shift toward flexible manufacturing capacity in industry to accommodate situations such as rapid pandemic response, variable production capacity planning for market demand of biosimilars, the desire to manufacture within closer proximity to affected population bases, and personalized medicine.
- GEN RoundupA panel of pharmaceutical industry experts discusses the advances in bioprocess filtration for improving virus removal and the key to economic large-scale filtration over the last decade.
- INTERPHEX New York 2016Meissner was interviewed at their booth during the INTERPHEX New York event to discuss advancements such as the BioLink® Modular Overmolding Technology, the Saltus® M200 Single-Use Mixing System, and the pharmaceutical industry’s most robust biocontainers – FluoroFlex® PVDF Biocontainers.
- American Pharmaceutical ReviewA panel of pharmaceutical industry experts delivers advice for first time adopters of single-use technology, and which processes are easiest to transition to disposable technologies.
- Continuous Quality VerificationThis article discusses an integrated quality/manufacturing approach to ensure the integrity of single-use biocontainers.
Fluid Thoughts
Fluid Thoughts Form