Introduction
The speed with which homogeneity is reached within solutions is a critical performance attribute of single-use mixing systems. These systems are commonly used in biopharmaceutical and pharmaceutical media and buffer preparation processes, often involving the hydration of a powder. Recirculation mixing performed using a peristaltic pump can provide a simple and cost effective alternative to more elaborate mixing systems such as those which incorporate a magnetically driven impeller.
This technical bulletin highlights the results of conductivity testing to characterize recirculation mixing performance of single-use systems deployed in Meissner’s QuaDrum® rigid outer containers (ROCs). All three standard QuaDrum® ROCs, with nominally rated volumes of 50 L, 100 L and 200 L, were evaluated with corresponding single-use systems. The scope of this testing included varying flow rates and mixing directions while using NaCl as the mixing solute.
Materials and Methods
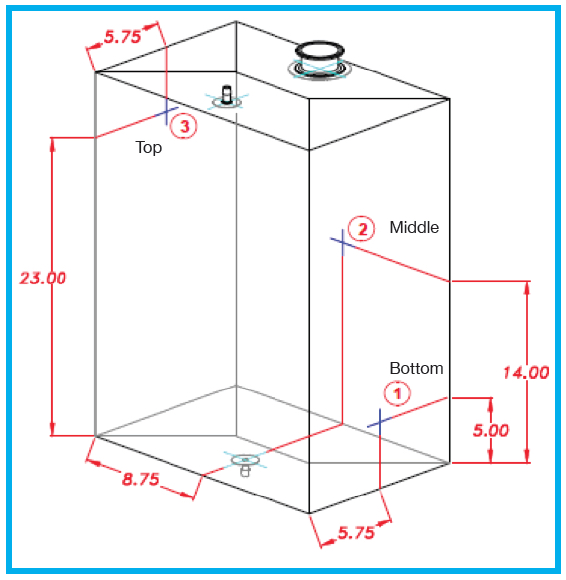
Electronic conductivity meters (Mettler Toledo InPro 7108-TC-VP and InPro 7100) were mounted and sealed into locations which allowed for data capture at the top, middle and bottom of 50 L, 100 L and 200 L biocontainers deployed in QuaDrum® ROCs (as
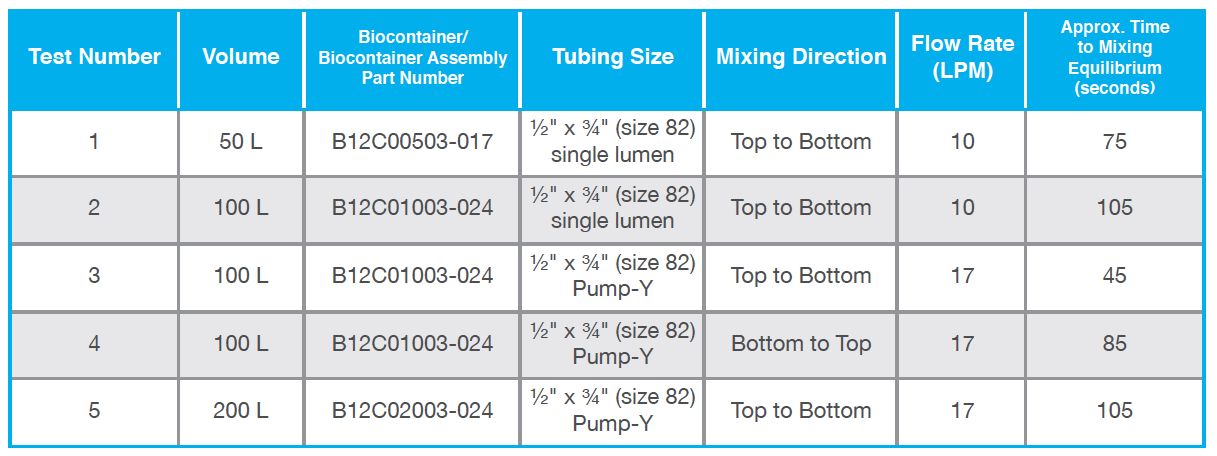
shown by the drawing in Figure 1). The QuaDrum® ROCs used for this testing included the optional bottom drain version (part numbers FASD-050B, FASD-100B and FASD-200B) which was installed on their corresponding accessory dollies to facilitate the use of a bottom mounted fluid path. A volume of water equivalent to the nominally rated capacity of each (i.e. 50 L, 100 L or 200 L) was added to each single-use assembly. The recirculation flow was established using a Masterflex® I/P peristaltic pump set to the desired rate that was verified via an offline measurement. Mixing direction was controlled based on the direction of recirculation flow through the assembly and was either top to bottom (i.e. fluid evacuated from the top of the closed system and returned through the bottom port) or bottom to top (i.e. fluid evacuated from the bottom of the closed system and returned through the top port). NaCl (VWR GR ACS Sodium Chloride) was introduced into the system through a large bore 3″ TC top port in the quantity necessary to achieve a concentration of 15 g/L for the given fluid volume. The system was allowed to mix until all three of the conductivity readings were stable. Five tests were conducted, which included analysis of all three fluid volumes with varying flow rates and mixing directions. The specific test conditions are presented in Table 1.
Results and Discussions
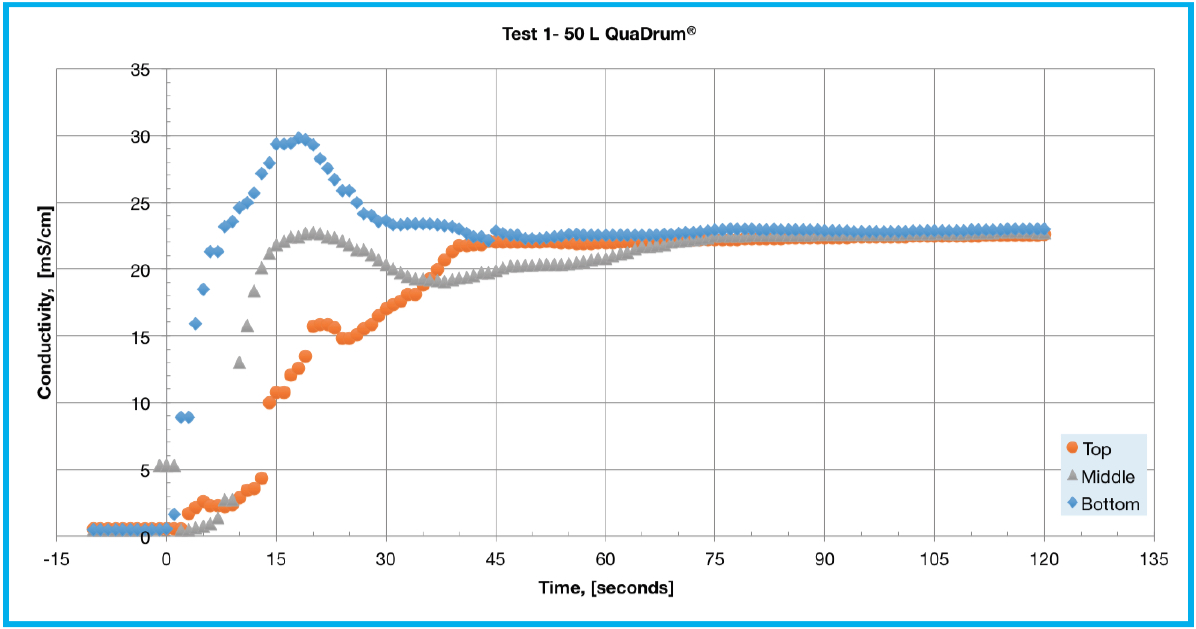
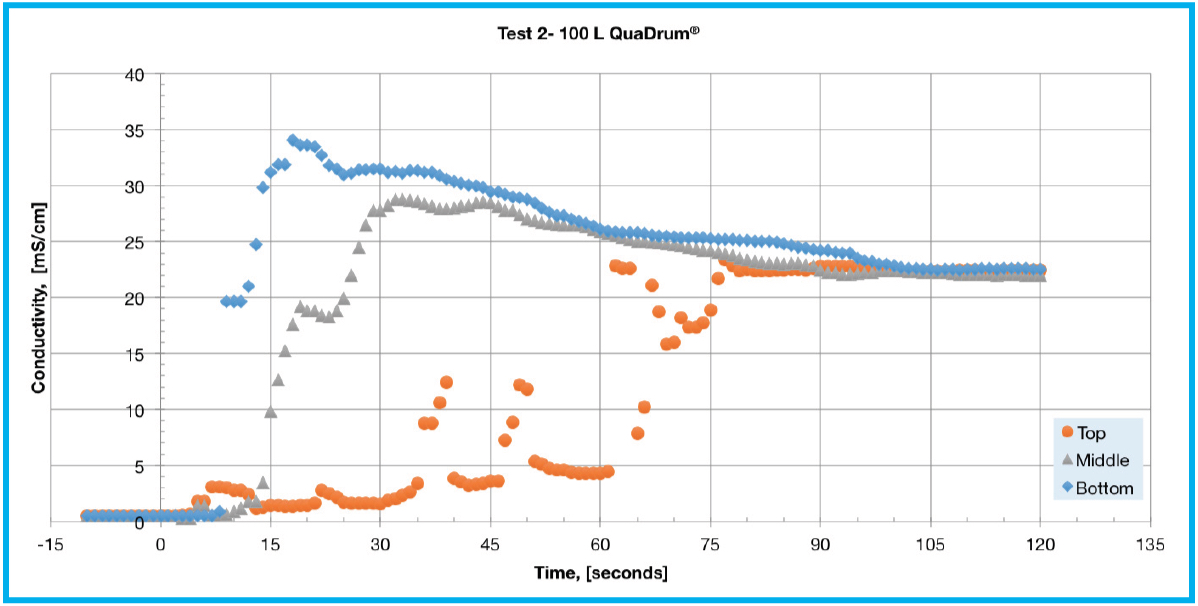
The conductivity testing results are presented in Table 1 and Figure 2. The response from the bottom sensor displayed a characteristic overshoot before settling to equilibrium for each of the test conditions. This can be explained due to initial NaCl accumulation near the bottom of the biocontainer immediately following solute addition. The middle level sensor also followed a similar response to that of the bottom sensor but with a smaller overshoot. The top sensor displayed a varied response based on the specific test being performed. During tests 1 and 4 the top sensor gradually approached the equilibrium conductivity value while during tests 2 and 5 it displayed a delayed response with a few spikes in conductivity. In Test 1 (Figure 2A), all three sensors reached equilibrium conductivity at about 75 seconds and follow the general patterns described above. Test 2 (Figure 2B), showed an unusual response from the top sensor, which spiked around 35, 47, and 63 seconds.
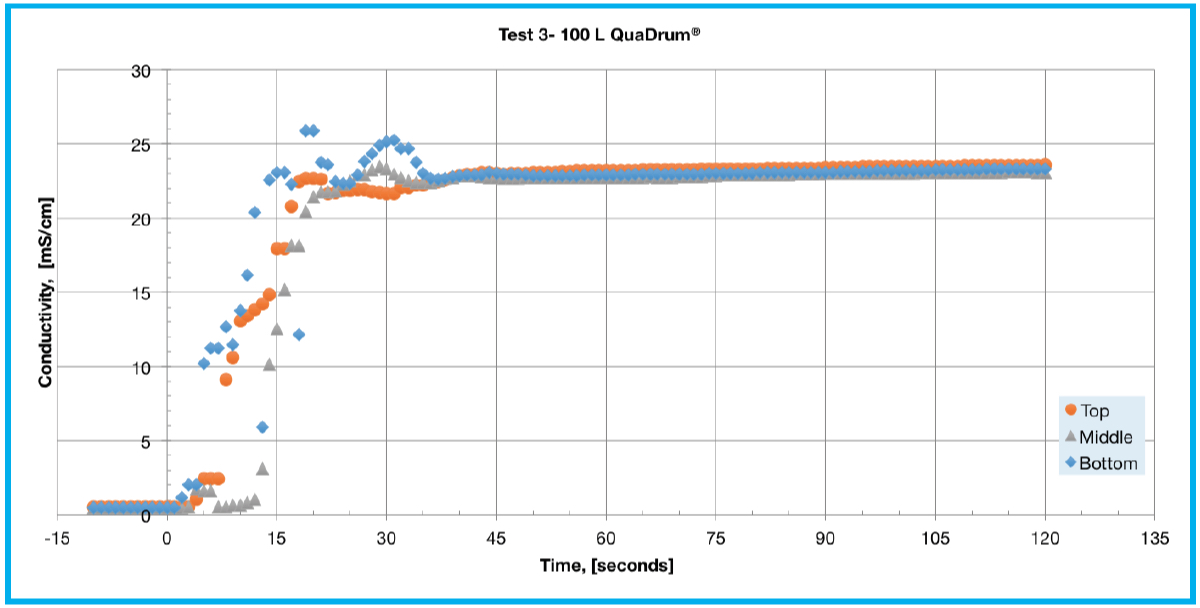
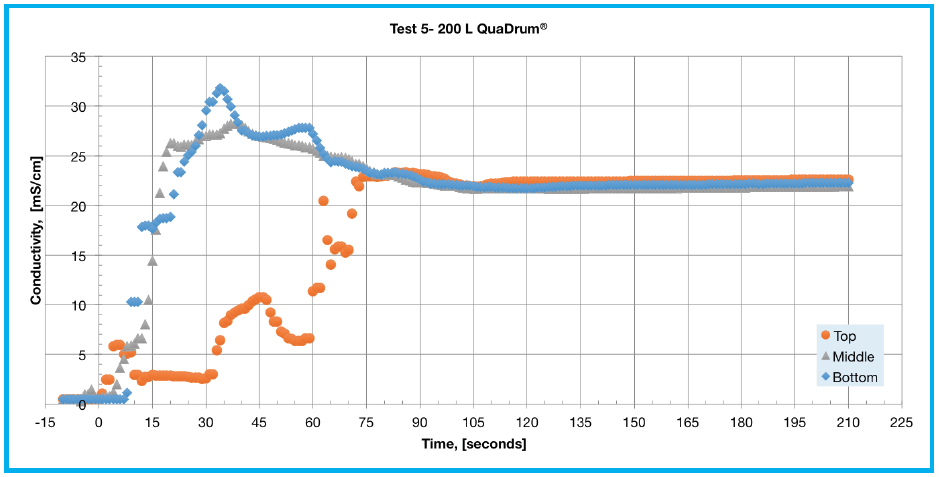
This is likely due to a relatively low flow rate for the larger liquid volume with a time to equilibrium of 105 seconds. Tests 3 and 4 (Figures 2C and 2D) examine the effect of recirculation flow direction on a 100 L single-use assembly deployed in a QuaDrum® ROC. These tests were performed using a pump-Y element, as opposed to a single lumen pump tubing segment, in order to achieve an increased flow rate of 17 LPM. The difference in performance is significant as it took 45 seconds to reach equilibrium for top to bottom recirculation, whereas bottom to top recirculation took 85 seconds. In Test 5 (Figure 2E), all three sensors reached equilibrium at around 105 seconds. The response of Test 5 appears similar to that shown in Test 2, with an overshoot at the bottom and middle with a delayed response at the top sensor. This is explained by the bottom becoming heavily concentrated while the top experienced mixed concentration levels. At a flow rate of 17 LPM, the 200 L mixing assembly takes a few minutes of mixing to reach a consistent concentration level.
Conclusion
The results of conductivity testing using QuaDrum® ROC based single-use mixing assemblies indicate that achieving an evenly mixed solution within a relatively quick period of time is possible using this technique. The testing shows the significance of using an appropriate flow rate predicated on the overall volume of the system. It further demonstrates that for powdered solutes with a specific gravity greater than one, employing a mixing direction that circulates fluid from the top of the system and returns it to the base, is preferable. Therefore, under the appropriate operating conditions, recirculation mixing using QuaDrum® ROCs with installed single-use biocontainer assemblies can provide an effective means to achieve and maintain homogenous solutions.
For more information or test data, please contact Meissner Filtration Products.