
Freeze & Thaw Champions
Meissner is the industry’s premier choice for critical freeze and thaw operations, delivering unmatched security and performance at every scale. Whether you’re working with small process development (PD) volumes or full commercial scale production, Meissner provides the most secure platforms on the market—removing risk at every processing step from fill and freeze to thaw and dispense. Count on our team of seasoned experts to help you optimize your freeze-thaw operations so you can focus on the delivery of medicines to the patients who count on you.
Unmatched Security
Our CryoVault® platform uses robust, rigid ¼” thick HDPE single-use containers to deliver maximum product protection and consistent freeze/thaw performance. Each CryoVault® container is engineered to provide industry-leading protection for high-value biopharmaceuticals during freeze, transport, and thaw operations. Our TepoFlex® platform uses one of the industry’s strongest polyethylene biocontainers to deliver secure freeze and thaw options in flexible containers up to 10 L.

Scalable & Flexible
Effortlessly scale from process development through commercial production in CryoVault® containers from 30 mL to 75 L, while maintaining process integrity and compliance with regulatory standards. Our modular systems allow flexible implementation tailored to your batch size and process needs.
End-to-End Integration
From sterile fill and controlled-rate freezing to automated handling, qualified shipping, and precise thawing through dispensing to downstream operations, Meissner’s CryoVault® platform minimizes manual manipulation—and consequently risk—at every step of processing. The CryoVault® platform’s integrated material handling solutions provide your team with a full suite of pre-qualified options—automated lifts, integrated pallets, and custom carts—to ensure safe, efficient, repeatable movement of frozen product throughout your facility and global supply chain.
Let's talk.
Send a message to our
industry experts.
Freeze & Thaw Champions
Freeze & Thaw Links

CryoVault® is the gold standard, providing critical product security from small volume to bulk commercial scale.
Superior Product Security: 1/4″ rigid HDPE container protects biologics in the industry’s most secure container.
Scalable Volumes: From stability containers with fill volumes of 30 mL and 100 mL and large scale containers with working volumes up to 75 L, CryoVault® delivers consistent performance from bench top to pilot to commercial scale.
Global Logistics Solution: Seamless handling, storage, and shipping—validated for global distribution.
Automated Handling: Pre-qualified lifts, carts, and pallets for safe, repeatable movement of contained product.
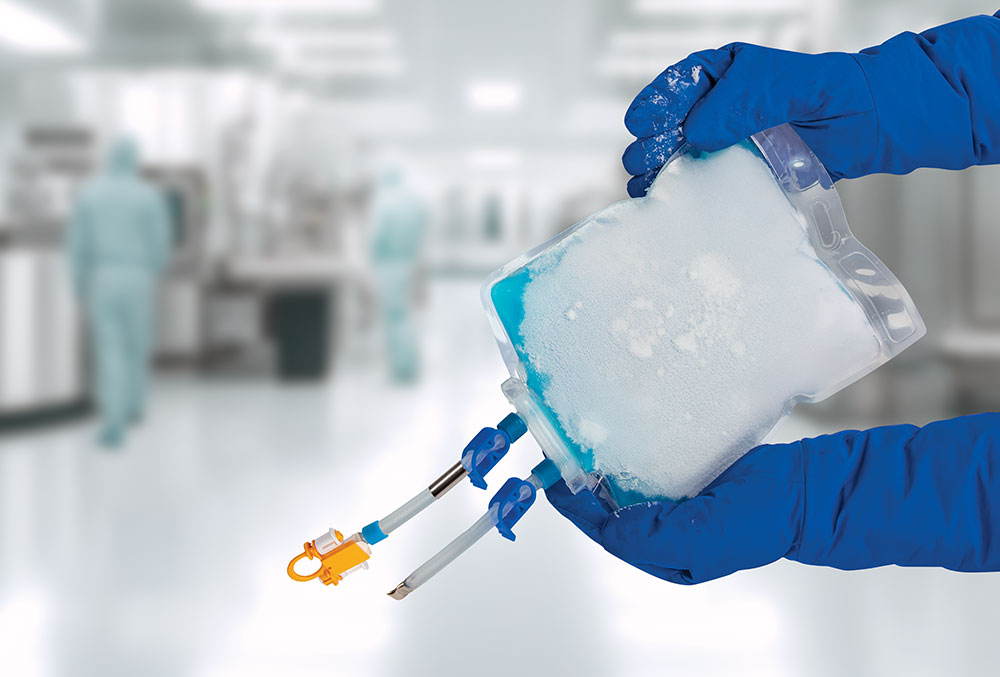
TepoFlex® provides the most secure flexible biocontainer options for volume requirements up to 10 L.
Flexible Implementation: The TepoFlex® biocontainer’s flexible single-use design can be integrated into existing infrastructure. It is compatible with a variety of freezers, including but not limited to conventional blast freezers as well as vertical plate freezers, enabling deployment across various stages of drug development and manufacturing without major capital investment.
Process Scalability: TepoFlex® is available to accommodate process sizes from 50 mL to 10 L with a secure biocontainer.
Exceptional Reliability: TepoFlex® biocontainers allow for highly reliable freezing during freeze/thaw cycles.

Freeze & Thaw Platform FAQs
Yes, our team of Applications Engineers can partner closely with you to design a Freeze/Thaw solution incorporating either CryoVault® rigid containers and/or TepoFlex® flexible container assemblies that meet your specific process requirements. We offer a library of standard assemblies as a starting point that can be adapted based on your workflow, volume range, and connectivity requirement for up and downstream integration.
Choosing the right Freeze/Thaw solution depends on several factors, including drug modality, fill volume, batch size, freezing method, and logistics, such as shipping and transport requirements. Our team works closely with you to assess both technical and operational risk factors and recommend a container solution that balances robustness, fitment with existing infrastructure, and scalability to meet your process and quality objectives.
Our team has over a decade of experience supporting global biopharma and Contract Development Manufacturing Organization (CDMO) clients in developing robust Freeze/Thaw strategies—from early-stage pre-clinical programs to commercial-scale production.
Our team of Freeze/Thaw experts are available to provide consultative support to help design a secure and reliable Freeze/Thaw solution. Our comprehensive documentation package may include sterilization validation, material certification, extractables and leachables data, and compatibility information.
Yes, you can order assemblies for trial to support feasibility studies that allow you to validate performance before committing to full-scale implementation.