Recirculation Flow Testing a FlexGro® Biocontainer Fitted with an Anchored Dip Tube for Perfusion Culture

Introduction
Perfusion processes are rapidly being adopted as an alternative to traditional batch and fed batch culturing of mammalian cells. In one type of perfusion system, a bioreactor is connected to a recirculation pathway where the exchange of spent media occurs. Rocker bioreactors, also commonly referred to as wave bioreactors, have been a popular choice for seed train applications and small scale protein production for required working volumes below the 100 L range. The continuous rocking motion of the reactor generates a wave inside of a single-use biocontainer, allowing aeration of the cell culture while avoiding problematic high shear conditions. However, one commonly encountered challenge of using rocker bioreactors in perfusion mode is the undesirable transfer of air into the recirculation pathway. These entrapped air bubbles compromise the effectiveness of the perfusion filter, which is usually accomplished via either a tangential flow filtration (TFF) or an alternating tangential flow filtration (ATF) device. This technical bulletin evaluates a solution to enable perfusion culture via a new anchored dip tube that was designed to prevent air migration into the recirculation pathway. A 50 L FlexGro® biocontainer connected to a recirculation loop was tested for the presence of entrapped air under varying processing conditions of liquid volume, rocking rate, and rocking angle. The experimental data provides insights into the relationship between liquid volume, rocking rate, and rocking angle, which is useful in defining the proven acceptable range (PAR) of processing parameters for the perfusion system.
Materials and Methods
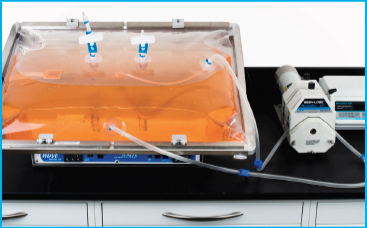
A Meissner 50 L FlexGro® biocontainer (part number B12R00505-006) modified to incorporate a ¼” x 7/16″ (ID x OD) anchored dip tube was mounted to a rocker bioreactor (GEHC, Wave Bioreactor 20/50 system), filled with dyed water, and inflated with air to approximately 1 psi, according to the manufacturers instructions. A recirculation flow pathway comprised of transparent silicone tubing was then established by interconnecting the inlet and outlet tubing. A peristaltic pump (Masterflex® I/P) was used to maintain a constant recirculation flow rate of 8 L/min. The testing setup is shown in Figure 1. Rocking angles of 6-10°, rocking rates of 15-35 rpm, and liquid volumes of 10-25 L were used in combination to determine whether air transfer into the recirculation pathway occurred. Table 1 summarizes the testing parameters. The recirculation pathway was visually inspected for migration of air bubbles for each of the operational combinations tested.

Results and Discussions
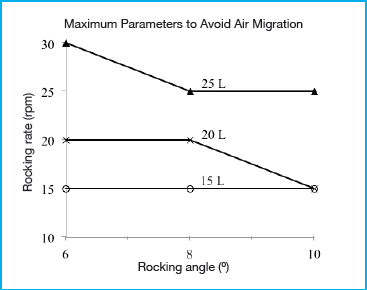
The graph presented in Figure 2 shows the maximum rocking rate determined as a function of rocking angle and working volume before air migration occurred. A recirculation flow rate of 8 L/min was used in order to represent worst case conditions and amplify the migration of air through the tubing. As hypothesized, the rocking angle, rocking rate, and working volume all had an effect on the transfer of air into the tubing. An increase in rocking angle and rocking rate contributed to increased air transfer, while a decrease in liquid volume also led to an increase in air transfer. To prevent air migration, meeting two conditions seemed to be crucial. First, the anchored dip tube must remain submerged below the liquid level at all times. Second, the liquid near the dip tube cannot contain air bubbles. All three operating parameters of volume, rocking angle, and rocking rate contributed to whether the first condition was met. Although the anchored dip tube was positioned along the centerline of the biocontainer, air entrapment still occurred under certain conditions, even when the highest working volume of 25 L was used. When the rocking rate was increased high enough (above 30 rpm for an angle of 8° or greater) the inertial forces generated caused the bulk of the wave to move beyond the centerline, resulting in a liquid level below the anchored dip tube inlet opening. The primary contributor to whether the second condition is met is the rocking rate. At higher rocking rates, increased agitation resulted in turbulent flow patterns, which produced air bubbles in the liquid. It is likely that this effect will be further exacerbated during actual cell culture due to the generation of foam. It is expected that the 50 L biocontainer used is representative of a worst case condition when compared to the smaller 20 L and 10 L FlexGro® biocontainers because the effect of the inertial forces in generating turbulent mixing and agitation is expected to decrease at lower volumes.
Conclusion
A 50 L FlexGro® biocontainer modified with a centerline dip tube to effect a recirculation loop supports its use in perfusion applications, provided that the operational culture conditions used remain within the PAR to avoid air entrapment. Further experimental evaluation may be required using actual cell culture conditions in order to define a normal operating range (NOR) of operational parameters suitable for perfusion culture. Therefore, the results presented in this technical bulletin should only serve as an initial guide towards the adoption of FlexGro® biocontainers for perfusion culture.
For more information or test data, please contact Meissner Filtration Products.