CryoVault® Freeze & Thaw Platform

Let's talk.
Send a message to our
industry experts.
CryoVault® Freeze Thaw Platform
Let's talk.
Send a message to our
industry experts.
CryoVault® Freeze Thaw Platform
CryoVault® is a scalable, robust, completely single-use freeze and thaw platform for biopharmaceuticals.
This end-to-end solution provides the industry leading implementation of this demanding unit processing operation.
Scalable
CryoVault® can be seamlessly scaled from process development through clinical to full scale commercial production. Consistency in freeze-thaw performance throughout the various scales of operation is a key performance attribute of the system.
The platform is not only scalable from a freeze-thaw performance perspective but also from an operational standpoint. CryoVault® scales operationally with your volume requirements by providing containers that can hold up to 75 L along with all the associated material handling infrastructure. The CryoVault® Bench Top Freezer supports process and product development for the comprehensive freeze and thaw platform, and uses minimal process fluids for testing and validation.
Robust
CryoVault® addresses the need to provide fluid integrity. The system uses robust rigid wall single-use assemblies to protect your product from fill to dispense. We understand the value of your critical biopharmaceuticals and this system provides the secure protection required.
The platform is based on robust single-use assemblies and an integrated infrastructure that provides process solutions to ensure the security of your product.
Single-Use
CryoVault® embraces the operational benefits associated with single-use systems. It is cost effective, simple to operate, clean, and can even be recycled.
CryoVault® Freeze & Thaw Platform Video
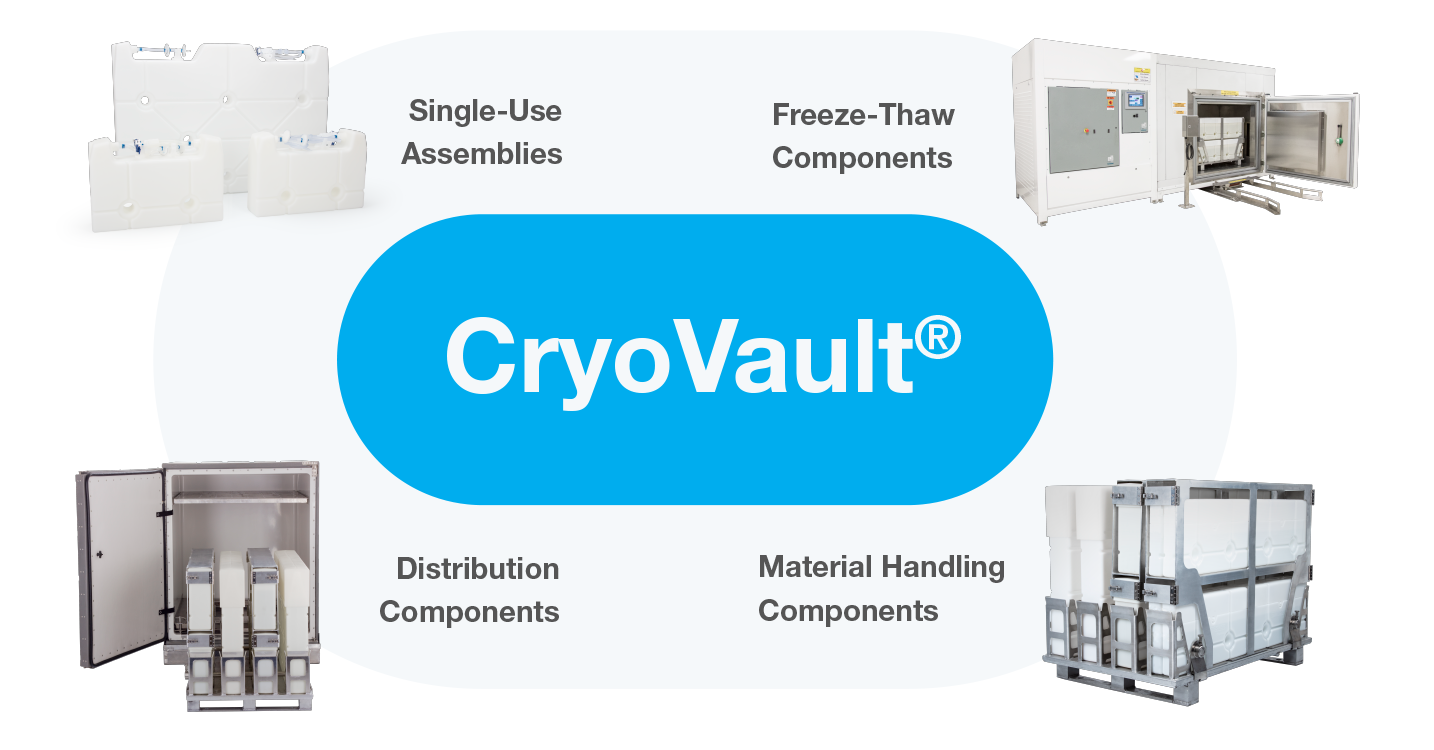
Modular Freeze and Thaw Platform
CryoVault® is a modular freeze and thaw platform composed of four components.

CryoVault® Freeze-Thaw Brochures
CryoVault® Standards Guide
CryoVault® FAQ
End-to-End Process Solution
The CryoVault® platform offers a comprehensive, end-to-end solution for your entire freeze-thaw process. It includes single-use assemblies, freeze/thaw systems, material handling infrastructure, and integrated shipping containers, ensuring robust performance from fill to dispense and every step in between.
Sterile/aseptic transfer to single‑use containers
Tailgate sampling
Controlled rate process
Automated handling equipment solutions
Frozen single‑use containers handled as a unitized load
Efficient space utilization using standardized footprint
Single‑use containers stored securely
Ships as unitized load
Form factor compatible with passenger aircraft
Controlled rate
Provision for agitation
Optional mixing performed without transfer
from single‑use freeze‑thaw container
Sterile/aseptic transfer to downstream processes
Low hold up volume
The CryoVault® platform provides a complete End-to-End solution (E2E) inclusive of BDS storage containers, freeze/thaw units, material handling infrastructure, and integrated shipping containers.
CryoVault® has been designed as a modular system to accommodate a high degree of flexibility for different batch volumes. All system components seamlessly integrate with each other. This allows the end-user to tailor the system implementation to their specific process needs. Meissner’s applications engineering team will assist in this process.
Single-Use Assemblies
The single-use assemblies are rigid HDPE containers with configurable fluid path assemblies referred to as top works. The containers feature a consistent freeze-path length throughout the different sizes to ensure process scalability. Single-use assemblies are currently available in the following volumes:
Process Development and Stability Scale—Maximum Fill Volume
- 30 mL
- 100 mL
- Bench top freezer – This small scale freeze and thaw solution allows for product and process development for the entire CryoVault® platform.
Clinical and Production Scale—Maximum Fill Volume
- 16 L
- 75 L
The clinical and commercial production scale single-use containers feature an integrated shroud that protects the fluid path assemblies during freeze-thaw, transport, and storage. Each assembly is individually serialized to provide material traceability. The rigid container component is manufactured from a medical grade HDPE resin, which has been rigorously tested, inclusive of the BPOG extractables protocol, and has a DMF. The largest container’s ¼” (6.4 mm) thickness provides exceptional robustness and a burst pressure exceeding 50 psi (3.4 bar), while the 16 L/20 L container has a burst pressure in excess of 100 psi (6.9 bar). Although burst pressure is only one facet of overall single-use system integrity, providing these values is a good indication of the industry step change in robustness that CryoVault® offers.
Material Handling Components
Material handling infrastructure is one of the most overlooked components of any single-use systems implementation, and it is especially critical for freeze-thaw applications. Meissner offers a wide variety of pre-qualified material handling solutions for CryoVault® at various scales of implementation. These include:
- Automated lifts and pallet handling solutions that facilitate programmable and repeatable material transfer between various process steps
- Integrated pallets that can accommodate up to 300 L of liquid in a euro-pallet footprint
- Carts designed specifically for transport, fill, and dispense operations



Freeze-Thaw Components
There are two primary clinical and commercial production scale freezer options for the CryoVault® platform. The large-scale freezer can accommodate up to four containers with a maximum fill volume of 75 L each, totaling a 300 L nominal working volume, or sixteen single-use containers of 16L each, totaling 256 L of nominal working volume, or any combination of 75 L and 16 L containers. The smaller scale freezer is available to accommodate up to a 96 L nominal working volume or six single-use containers of 16 L. Both freezers are programmable to provide fully controlled freeze and thaw cycles. Agitation, provided via gentle oscillation, is available for thawing sensitive proteins and expediting the thaw process.

Distribution Components
Shippers, which are designed to provide robust distribution performance, are a key component of the CryoVault® platform. These shippers are offered in both single-use and reusable options to optimize operations depending on the network setup of the drug substance and drug product manufacturing sites. Both are designed to ship on commercial aircraft, which provides more transportation options than those that can only fit on dedicated freight airplanes. These shippers have been tested per ASTM D4169 and ISTA 3E protocols.



CryoVault® Freeze & Thaw Platform FAQs
CryoVault® is a next generation, single-use freeze/thaw platform, primarily directed at critical high value process fluid such as bulk drug substance. It provides a real change in terms of scalability and robustness compared to current solutions.
There was an industry need for a freeze-thaw solution that was single-use, robust (offered greater security than existing systems), and was scalable to large volume, particularly with the proliferation of decentralized Drug Substance (DS) and Drug Products (DP) processing sites.
Cryovessels have been the predominant technology. However, with recent adoption of single-use systems, new technologies such as biocontainer assemblies (bag assemblies) with outer support containers are available for freeze-thaw applications.
The advantage of a cryovessel system is that you can process large batches. The disadvantage, or area for improvement, is that the cleaning, sterilization, and validation of these systems require extensive infrastructure and labor to maintain.
Single-use film-based products have all the advantages of single-use but there can be integrity issues during freeze and thaw. Thin films are fragile and susceptible to tears during material handling processes. In addition, scalability is a big challenge as the largest volume options include 16 L maximum fill volume per system. Large scale BDS volumes require proper material handling throughout the freeze-thaw cycle.
CryoVault® is an end-to-end (E2E) platform solution that addresses the common challenges associated with bulk drug substance freeze-thaw operations. CryoVault® containers feature a rigid wall with customizable top works that, together, create a complete single-use assembly. The storage containers are modular in size and are available in maximum fill volumes of 75 L, 16 L, 5 L, and 1 L to provide operational flexibility.
CryoVault® is a single-use system for controlled freeze and thaw with all the benefits of single-use and all the features and robustness that you would get with a more traditional rigid cryovessel.
The key differentiator is the science behind the container design. There was intentional design around how bulk drug substance freezes and thaws. It was important to create consistent freeze path lengths, which is easier to control in a rectangular design than in a circular shape like a carboy.
Controlling the last point of freeze has a direct correlation to product integrity by preventing cryoconcentrations, and container integrity by preventing pressure buildup due to ice bridging. Consistent freeze path lengths permit scalability as more BDS can be put into larger containers while freeze-thaw performance remains the same. This provides a faster freeze-thaw cycle, which is critical from an operations perspective.
CryoVault® has been adopted by over 20 locations globally. Our clients realize that this is a unique solution for an obviously critical process step, and rightly so, as there is a high level of vetting by potential users.
Most single-use systems contain multiple polymers, which makes recycling nearly impossible. CryoVault® containers are made from a single plastic material, HDPE, which is a very recyclable material accepted by most waste streams. Of course, there must be a decontamination step first, but companies can work with their municipalities to explore recycling options.
The CV300 platform allows for batch freeze and thaw of up to 300 L of BDS using containers with maximum fill volumes of 75 L, 16 L, and 5 L for enhanced operational flexibility. Similarly, the CV96 platform allows for batch freeze and thaw of up to 96 L of BDS using both the 16 L and the 5 L maximum fill volume containers. Each of the CV300 and CV96 platforms feature their own freeze-thaw units and material handling equipment.
Download the CryoVault® Standards Guide to view product specifications.



